Safety Information
This topic provides the safety information for the system.
CE Marking Information
The
Centricity Perinatal product bears the CE Mark

indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive.
The country of origin can be found on the product label.
CAUTION: U.S. Federal law restricts this device to sale by or on the order of a physician.
List Of Standards
The following is a list of standards with which the device complies.
Full Standard Number | Standard Title | Standard Revision or Issue Date |
EN 62304 | Medical device software – Software life cycle processes | 2006 |
EN 62366 | Medical Devices – Application of usability to medical devices | 2008 |
General Safety Information
CAUTION: Customer-Supplied Hardware—Hardware supplied by the customer must meet the minimum specifications listed in the Centricity Perinatal Customer-Supplied Equipment Reference Manual.
The following table defines symbols used throughout this document and/or the product software:
Symbol | Definition |
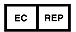
| This symbol indicates the AUTHORIZED REPRESENTATIVE IN THE EUROPEAN COMMUNITY of the product. |
| This symbol indicates the MANUFACTURER of the product. |
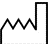
| This symbol indicates the DATE OF MANUFACTURE of the product. |
| This symbol indicates that the operator should CONSULT INSTRUCTIONS FOR USE for further information. |
| This symbol indicates the manufacturers BATCH CODE, or lot number of the product. |
| This symbol indicates conformity with the provisions of the Council Directive 93/42/EEC, concerning medical device and fulfills the essential requirements of Annex I of this directive. |
Rx Only | For products distributed in the US, the symbol for "Rx Only" indicates: Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner. |
| This symbol indicates that instructions for use are supplied in electronic form. |
Surveillance and Monitoring Concerns
CAUTION: Physiological Monitor Synchronization—Users and members of the Biomedical Engineering staff should be aware that the time on the monitor should be synchronized with the time on the systems' servers.
Erroneous Data Entry and Calculations
NOTE: Data Entry—Be sure that the specified patient is selected before performing data entry. Failure to make the selected patient the focal point of data operations could result in the data being placed into another patient file.
NOTE: Typographical Errors—Although the systems' modules may be configured to ensure that numeric data (for example, pulse, BP, or temperature) is within a specified range of acceptability, users may make typographical errors when entering data. Failure to verify your manually entered data could result in the storage of incorrect data.
System Security Considerations
NOTE: System Security—The GE Centricity products provide the capability to define the degree of user access to workstations through the use of IDs and passwords. It also allows the assignment of READ/WRITE privileges via security objects to determine who may access or record patient data. Failure to properly configure the security feature could result in unauthorized personnel gaining entry into Centricity and ultimately viewing or recording patient data.
©
2019 General Electric Company. All rights reserved. All information is
subject to change without notice. This information is the confidential and
proprietary information of General Electric Company. Unauthorized duplication
is strictly prohibited.
Centricity Perinatal Web | | | | | | |
 indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive.
indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive. indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive.
indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive.